House Minority Leader Joseph Stephen “Caraps” Paduano finds the Emergency Use Authorization (EUA) issued by the Food and Drug Administration to Sinovac “half-baked”.
In a press statement issued to the media, a copy of it was provided to Negros News Online, the Abang Lingkod Partylist solon said that while FDA has issued an EUA to Sinovac, yet they also issued an advice against administering it to senior citizens and health frontliners.
These contradicting statements had cast doubts on the efficacy of the vaccine, he stressed as he urged FDA to issue a clarificatory statement soonest.
The Minority Leader from Bacolod scored the agency for allegedly putting the image of Sinovac vaccine in a bad light which, in effect, cast doubts on its efficacy.
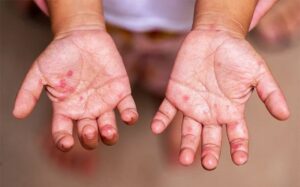
It’s very complicated because according to FDA Director General Eric Domingo, the vaccine is 91 percent effective in Turkey, 65.3 percent in Indonesia but it registered only 50.4 efficacy among medical frontliners in Brazil, he said, adding that regardless of the conflicting results of clinical trials, the fact remains that FDA had issued an EUA to Sinovac.
Rather than shed light on the use of the vaccine, the FDA has further eroded public trust and confidence in inoculation, and is not helping the government’s mass vaccination program, Paduano lamented.
In a briefing, FDA said that Sinovac’s efficacy rate ranged from 65.3% to 91.2%; however, its efficacy rate among healthcare workers is only at 50.4%, making it not the best option for them.