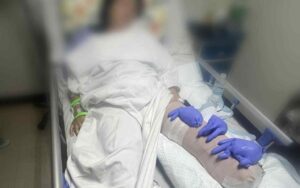
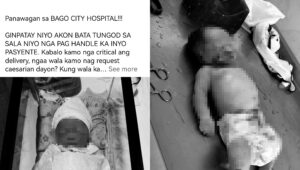
The Pfizer-BioNTech COVID-19 vaccine has been approved under Emergency Use Authorization (EUA) in the Philippines to reduce risk against COVID-19 for priority groups aged 16 and older, according to the Health Technology Assessment Council (HTAC).
Messenger RNA (mRNA) vaccines like the Pfizer-BioNTech COVID-19 Vaccine carry instructions for cells to make the “spike” protein unique to SARS-CoV-2, HTAC explains. It added, these vaccines teach the cells to make specific parts of the virus, allowing our bodies to detect these parts of the virus like it were the real virus, produce antibodies against them and fight off future infection.
Pfizer-BioNTech COVID-19 vaccine under EUA
The Food and Drug Administration (FDA) cleared the Pfizer-BioNTech COVID-19 Vaccine for emergency use on 14 January 2021. This emergency authorization speeds up the approval process for vaccines which normally takes years depending on how long clinical trials take.
Based on current available data, the Pfizer-BioNTech COVID-19 Vaccine can prevent symptomatic COVID-19, HTAC said in its advisory. Compared to experience with previous (non-COVID) vaccines, health authorities worldwide have noted few reported side effects, with no serious injuries or deaths resulting from vaccination. Researchers continue to monitor the vaccine’s long-term effectiveness and safety, and its ability to reduce the number of individuals experiencing symptoms and contracting severe disease.
Similarly, more evidence needs to establish the vaccine’s value for money, and its possible implications for reducing out-of-pocket expenses. Aside from these factors, HTAC also considers potential health, economic, and social benefits in recommending COVID-19 vaccines.
Based on existing evidence from ongoing trials, the HTAC has determined that the vaccine’s positive health impacts outweigh its short-term risks.
As new information emerges, the Council will continue to update its recommendations and provide our policy-makers, health providers, and the Filipino public evidence-based guidance.
Read the Evidence Summary: http://bit.ly/ESbiontechC19Pfizer
Read the guidance for healthcare providers: https://hta.doh.gov.ph/2021/02/09/pfizer-biontech-covid-19-vaccine/
The Health Technology Assessment Council (HTAC) is an independent advisory body created under the Republic Act 11223, otherwise known as the Universal Health Care Act, with the overall role of providing guidance to the Department of Health (DOH) and the Philippine Health Insurance Corporation (PhilHealth) on the coverage of health interventions and technologies to be funded by the government. It is the mandate of the Council to undertake technology appraisals by determining their clinical and economic values in the Philippine healthcare system, with the aim to improve overall health outcomes and ensure fairness, equity, and sustainability of coverage for all Filipino citizens.